What is Cushing’s Syndrome versus Cushing’s Disease?
Cushing’s syndrome is the result of an over-production of cortisol from the adrenal glands that make more cortisol than is needed by the body.
If the cortisol over-production is the result of over stimulation of the adrenal glands by the pituitary gland, the result is Cushing’s Disease.
Higher than normal cortisol level is also called hypercortisolism.
How is Cortisol produced?
How is Cortisol Produced?
Cortisol is produced by a specialized layer of cells in the zona fasciculata or outer layer (adrenal cortex) of the adrenal glands.
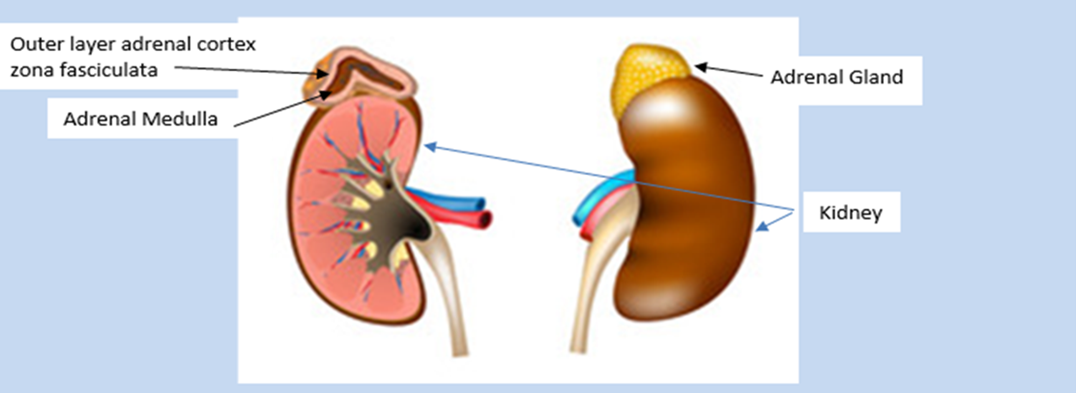
The adrenal glands are triangular shaped, about 5cm (3 inches) long and 2 cm (½ inch) high, weight about 4-5 grams each and sit above each kidney.
Cells in the hypothalamus and pituitary sense low levels of cortisol in the blood. The hypothalamus releases corticotropin releasing hormone (CRH) which travels down the pituitary stalk (infundibulum) to the pituitary gland instructing the corticotrope cells of the pituitary to produce adrenocorticotrophic hormone (ACTH). ACTH is released into the blood stream and circulates to receptors * in the outer layer or zona fasciculata of the adrenal gland where it sets in motion a series of chemical reactions and genetic transcription that ultimately produce cortisol.
- A receptor acts like a portal into cells. In this case, the ACTH is the key, and the receptor is the lock. Once the ACTH attaches to the receptor it opens the portal and enters the cell.
Control of Cortisol Hormone Production
Control of Cortisol Hormone Production
ACTH stimulates both the production and subsequent release of cortisol from the zona fasciculata of the adrenal cortex into the bloodstream.
Cortisol circulates to almost every tissue, organ and cell in the body. It prepares the body to defend itself against danger, spiking to higher levels as needed for flight or for fight. The threat may be internal such as from infection, illness, or injury, or external such as from an environment threat (perceived or real).
Cortisol: increases blood pressure, heart rate and respiratory rate; stimulates the liver to make glucose (a simple sugar for energy); converts proteins and carbohydrates into muscle energy.
ACTH release not only stimulates cortisol production but also aldosterone production and release from a separate zone of the outer cortex of the adrenal glands. Aldosterone is an essential hormone that alerts the kidneys to reabsorb sodium and water, increase the volume of circulating blood and blood pressure to deliver essential supplies to cells for energy and cell repair. This may be needed to add in the event of blood loss or injury or the need for ‘flight or fight’.
Adrenaline (epinephrine) and norepinephrine are known as catecholamines. Manufactured in the adrenal medulla or ‘centre portion’ catecholamines serve as the body’s alert system. Activated by the ACTH as a response to stress, these hormones also increase cortisol levels, constrict blood vessels, increasing blood pressure, heart rate and glucose levels to assist the body to adapt to or escape from a stressor.
The low or basal amount of CRH, ACTH and cortisol is produced by the body over each 24-hour period to keep the body in a stable or homeostatic state. At the onset of sleep cortisol levels reach their lowest in the early hours of the morning. This signals the hypothalamus to release CRH, stimulates ACTH to rises and turns on the production of cortisol. This is known as a positive feedback mechanism (+ve). (Figure 1). Although during the day demand for cortisol varies with different stressors, cortisol levels slowly decline until the low levels allowing the onset of sleep. This daily cycle is known as a circadian rhythm. (Figure 2).
After cortisol receptors are full, cortisol circulating in the blood reaches a threshold level which provides a negative feedback (-ve) signal the hypothalamus and pituitary to scale back production of CRH and ACTH completing the circadian cycle.
Figure 1 Feedback mechanism of cortisol, CRH and ACTH production:
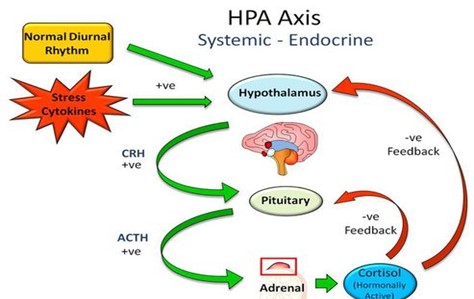
Figure 2: https://slideplayer.com/slide/8073009/:
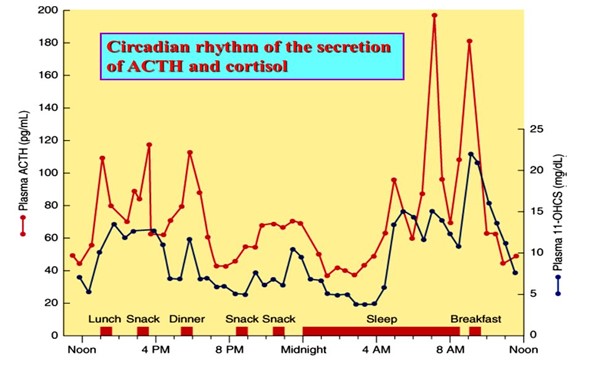
24 hour circadian rhythm typical of cortisol and ACTH production with late night low concentrations and early morning high concentrations.
Hypercortisolism, Cushing’s Syndrome and Disease
Hypercortisolism, Cushing’s Syndrome & Disease
Hypercortisolism is a state of more cortisol than needed by the body. This may be the result of medications given for the treatment of another disease or from increased production in the body.
Cushing’s syndrome is a disease state of excess or over production of cortisol.
There are 3 forms of Cushing’s syndrome:
- Cushing’s syndrome: Cells of the zona fasciculata of adrenal glands make too much cortisol from a tumor in the adrenal gland. This is autonomous production without the need for stimulation from ACTH and is known as ACTH independent Cushing’s.
- Cushing’s disease: Corticotrope cells in the pituitary gland make too much ACTH, which, in turn, tells the adrenal glands to make too much cortisol. This is usually the result of a pituitary tumor or adenoma and is known as ACTH dependent Cushing’s.
- Ectopic Cushing’: Rarely, an ACTH producing tumor is found in other parts of the body such as the lung.
Cushing’s remains a rare disease which is diagnosed in children and adults, and more commonly in women than men. Genetic mutations that enhance the ACTH and cortisol producing cell multiplication or resist negative feedback mechanisms are being studied but the cause of such mutations is not specifically known. Cushing’s disease itself is not known to be inherited.
Diagnostic Testing and Evaluations
Diagnostic Testing and Evaluations
To confirm hypercortisolism and determine the source of excess ACTH and/or cortisol usually requires multiple tests. These include:
- A random blood draw for cortisol and ACTH: Best drawn early in the morning but often not adequate for diagnosis because of normal variability in levels. A low ACTH level and a high cortisol level suggests adrenal source. A high ACTH suggests a pituitary source of cortisol.
2. Collection of a 24-hour urine free cortisol level (2 tests).
Normal: Below the upper limit of normal for laboratory norms

3. Collection of salivary cortisol samples at bedtime (2 tests):
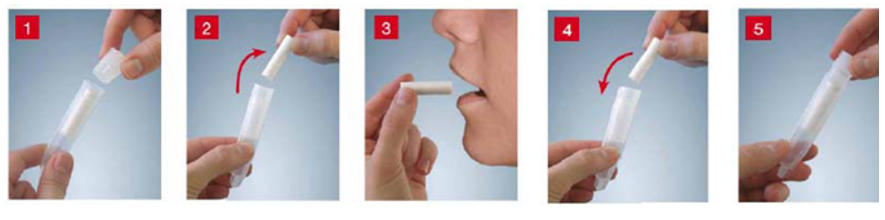
4. An oral/ overnight or 2-day dexamethasone suppression test:
Normal: less than 1.8 micrograms per deciliter (mcg/dL) or 50 nanomoles per liter (nmol/L).
5. Magnetic Resonance Imaging (MRI) of the brain and pituitary gland.
6. An Ipsilateral Sinus Sampling (IPSS) or Cavernous Sinus Sampling (CSS) To determine site of hypersecretion in the pituitary. (See Reference: Zampetti et al.).
7. Octreotide scan if ectopic Cushing’s is suspected.
8. An abdominal CT scan if adrenal Cushing’s syndrome is suspected.
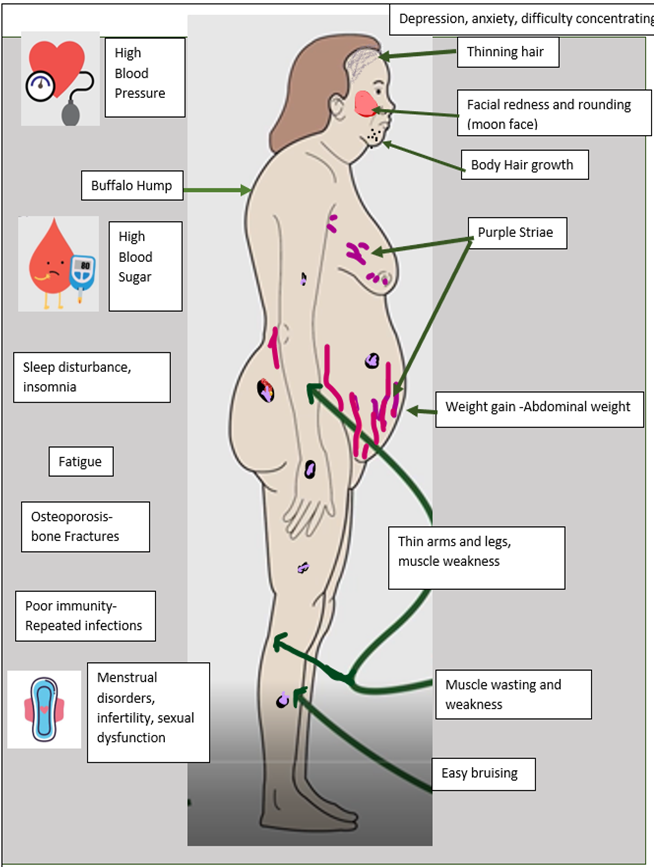
Signs and Symptoms of Cushing’s
Signs and Symptoms of Cushing’s
Treatment of Cushing’s Disease (treatment 1 and 2)
Treatment of Cushing’s Disease
1.Pituitary Surgery
First line therapy is the surgical removal of the pituitary tumour, if one is evident on MRI. Studies have shown that a tumour may not be seen in up to around 50% of patients with signs, symptoms and testing positive for Cushing’s disease. Procedures such as the IPSS, CSS and octreotide and nuclear scans may be helpful to identify the location of an ACTH tumour. The goal of surgery is to remove the tumor and restore the normal circadian CRH, ACTH and Cortisol production.
Post-operative treatment
Immediately after surgery, levels of cortisol and ACTH in the blood will be measured, usually at midnight and early in the morning. If levels are low, the patient will be prescribed hydrocortisone or a similar glucocorticoid medication that is continued after discharge from the hospital. The dose of this glucocorticoid will be determined according to pre and post operative symptoms and post operative cortisol and ACTH levels. The dose is slowly tapered, or withdrawn, until a cortrosyn stimulation test is performed to confirm normal adrenal production of cortisol. Medication withdrawal may take weeks or many months.
Symptoms of relative adrenal insufficiency may be experienced during withdrawal. These may include fatigue, headache, nausea, dizziness, abdominal discomfort, or loose stools. If severe, extra hydrocortisone or glucocorticoid is recommended until symptoms subside.
As the cortisol level falls, blood pressure, blood sugar, weight etc begin to decrease. This requires regular monitoring and a close working relationship between patient, endocrine team and general (primary care) practitioner to adjust medications for blood pressure and diabetes mellitus as needed
Further testing will be done after the glucocorticoid is stopped to ensure there is no further excess cortisol being produced. The lifetime chance of recurrence of Cushing’s disease is estimated at around 30%.
- Medical Therapy
If surgery is not recommended, or fails to achieve normal cortisol production, medical therapies can be used. This may be in the form of injections or oral medications such as tablets or capsules.
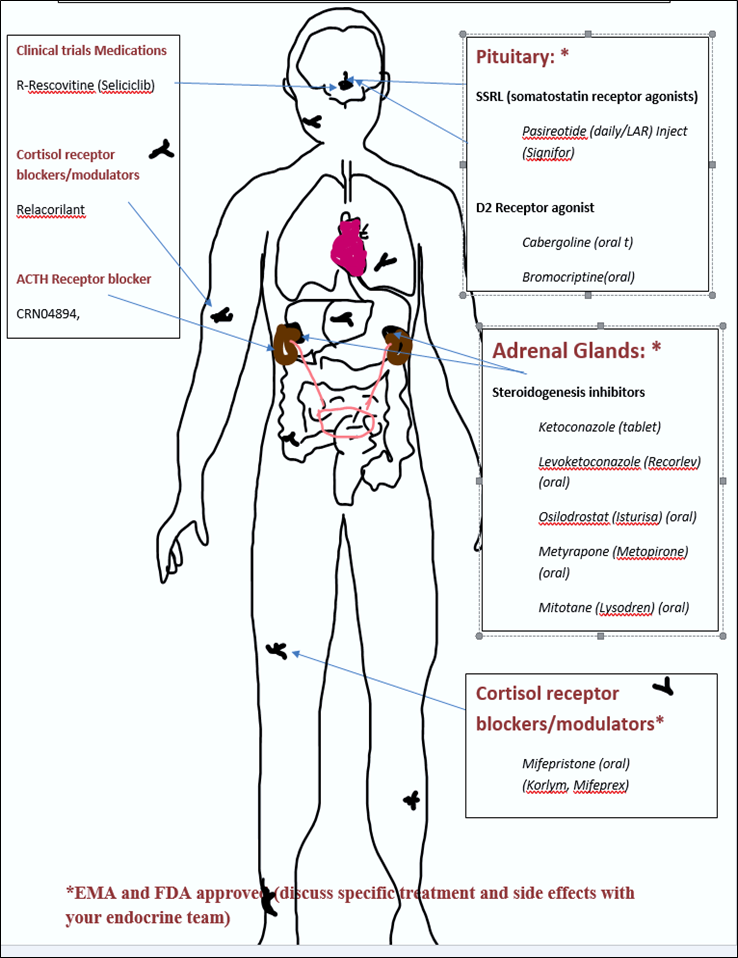
3. Medication Target (see seperate chapter)
4. Adrenal Surgery (see seperate chapter)
Current Medications Target (treatment 3)
3. Current medications target
- Receptors in the pituitary gland to stop ACTH production: called somatostatin or ligands (SRLs)
- Specific steps in the process of the manufacture of the steroid cortisol: called steroidogenesis inhibitors. These may block ACTH from acting on the cells of the adrenal cortex or block the conversion of inactive forms to active forms of cortisol.
- Cell receptors around the body to block the attachment of cortisol and stop cortisol from acting on body cells: called cortisol receptor blockers or modulators.
Adrenal Surgery (treatment 4)
4. Adrenal Surgery
If pituitary surgery is unsuccessful, and medical therapies fail to control the cortisol excess, one or both adrenal glands can be surgically removed. If both adrenal glands are removed synthetic cortisol such as hydrocortisone replacement is needed life-long for surgical Adrenal Insufficiency. Mineral corticosteroid in the form of fludrocortisone is also given to replace the action of aldosterone in balancing body electrolytes and fluid volume.
ACTH levels will be monitored regularly along with symptoms of Nelson’s syndrome such as skin darkening. Brain MRI will also be monitored for any pituitary tumour regrowth.
Abbreviations
Abbreviations
CRH Corticotropin Releasing hormone
ACTH Adrenocorticotropin hormone
HPA Hypothalamic, Pituitary, Adrenal Axis
AI Adrenal Insufficiency
SAI Secondary adrenal insufficiency
MRI Magnetic Resonance Imaging
IPSS Ipsilateral Sinus Sampling
CSS Cavernous Sinus Sampling
UFC Urine Free Cortisol
DEX/CRH Dexamethasone/Corticotropin Releasing Hormone
References
REFERENCES
Oster H, Challet E, Ott V, Arvat E, E. de Kloet R, Dijk D-J, Lightman S, Vgontzas A, Van Cauter E. (2017The Functional and Clinical Significance of the 24-Hour Rhythm of Circulating Glucocorticoids, Endocrine Reviews, 38, (1) Pages 3–45, https://doi.org/10.1210/er.2015-1080
Lonser, R. R., Nieman, L., & Oldfield, E. H. (2017). Cushing’s disease: pathobiology, diagnosis, and management, Journal of Neurosurgery JNS, 126(2), 404-417. Retrieved Nov 8, 2022, from https://thejns.org/view/journals/j-neurosurg/126/2/article-p404.xml
https://rarediseases.org/rare-diseases/cushing-syndrome/
Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008 May;93(5):1526-40. http://www.ncbi.nlm.nih.gov/pubmed/18334580
Newell-Price, J., Nieman, L. K., Reincke, M., & Tabarin, A. (2020). ENDOCRINOLOGY IN THE TIME OF COVID-19: Management of Cushing’s syndrome, European Journal of Endocrinology, 183(1), G1-G7. Retrieved Nov 8, 2022, from https://eje.bioscientifica.com/view/journals/eje/183/1/EJE-20-0352.xml
Zilio M, Barbot M, Ceccato F, Camozzi V, Bilora F, Casonato A, Frigo AC, Albiger N, Daidone V, Mazzai L, Mantero F, Scaroni C. Diagnosis and complications of Cushing’s disease: gender-related differences. Clin Endocrinol (Oxf). 2014 Mar;80(3):403-10. doi: 10.1111/cen.12299. Epub 2013 Sep 4. PMID: 23889360.
Nicolaides NC, Charmandari E, Kino T and Chrousos GP (2017) Stress-Related and Circadian Secretion and Target Tissue Actions of Glucocorticoids: Impact on Health. Front. Endocrinol. 8:70. doi: 10.3389/fendo.2017.00070
Zampetti, B., Grossrubatscher, E., Dalino Ciaramella, P., Boccardi, E., & Loli, P. (2016). Bilateral inferior petrosal sinus sampling, Endocrine Connections, 5(4), R12-R25. Retrieved Nov 9, 2022, from https://ec.bioscientifica.com/view/journals/ec/5/4/R12.xml
https://www.kch.nhs.uk/Doc/pl%20-%20933.1%20-%20overnight%20dexamethasone%20suppression%20test.pdf
Pivonello R, Ferrigno R, De Martino MC, Simeoli C, Di Paola N, Pivonello C, Barba L, Negri M, De Angelis C, Colao A. Medical Treatment of Cushing’s Disease: An Overview of the Current and Recent Clinical Trials. Front Endocrinol (Lausanne). 2020 Dec 8;11:648. doi: 10.3389/fendo.2020.00648. PMID: 33363514; PMCID: PMC7753248.
Author: Chris Yedinak DNP, FNP
Associate Professor
Oregon Health & Sciences University Portland. OR. USA
Updated November 2022